Cynnwys
- Main points
- Overview
- Approach 1: Prevalence of any symptom at a point in time after infection
- Approach 2: Prevalence of continuous symptoms after infection
- Approach 3: Prevalence of self-reported long COVID
- Comparison with other studies
- Updated estimates of the prevalence of post-acute symptoms among people with coronavirus (COVID-19) in the UK, 26 April 2020 to 1 August 2021 data
- Glossary
- Data sources and quality
- Related links
1. Main points
Experimental estimates of the prevalence of symptoms that remain 12-weeks after coronavirus (COVID-19) infection (commonly referred to as "long COVID") range from 3.0% based on tracking specific symptoms, to 11.7% based on self-classification of long COVID, using data to 1 August 2021.
This analysis focusses on the number of Coronavirus Infection Survey (CIS) participants with post-acute symptoms out of those with laboratory-confirmed COVID-19, unlike our monthly prevalence publication where the denominator is the national population (irrespective of infection).
Approach 1: Prevalence of any symptom at a point in time after infection. Among study participants with COVID-19, 5.0% reported any of 12 common symptoms 12 to 16 weeks after infection; however, prevalence was 3.4% in a control group of participants without a positive test for COVID-19, demonstrating the relative commonness of these symptoms in the population at any given time.
Approach 2: Prevalence of continuous symptoms after infection. Among study participants with COVID-19, 3.0% experienced any of 12 common symptoms for a continuous period of at least 12 weeks from infection, compared with 0.5% in the control group; this estimate of 3.0% is based on a similar approach to the one we published in April 2021 (13.7%), but is substantially lower because of a combination of longer study follow-up time and updated statistical methodology. The corresponding prevalence estimate when considering only participants who were symptomatic at the acute phase of infection was 6.7%.
Approach 3: Prevalence of self-reported long COVID. An estimated 11.7% of study participants with COVID-19 would describe themselves as experiencing long COVID (based on self-classification rather than reporting one of the 12 common symptoms) 12 weeks after infection, and may therefore meet the clinical case definition of post-COVID-19 syndrome, falling to 7.5% when considering long COVID that resulted in limitation to day-to-day activities; these percentages increased to 17.7% and 11.8% respectively when considering only participants who were symptomatic at the acute phase of infection.
Irrespective of the approach to measurement, post-acute symptom prevalence was highest in females, adults aged 50 to 69 years, people with a pre-existing health condition, and those with signs of high viral load at the time of infection.
If you are worried about new or ongoing symptoms four or more weeks after having COVID-19, there are resources available to help: see the NHS webpage on the long-term effects of coronavirus and the Your COVID Recovery website, which can help you to understand what has happened and what you might expect as part of your recovery. The time it takes to recover from COVID-19 is different for everyone, and the length of your recovery is not necessarily related to the severity of your initial illness or whether you were in hospital.
This is analysis of new, recently collected data, and our understanding of it and its quality will improve over time. Long COVID is an emerging phenomenon that is not yet fully understood. The estimates presented in this release are experimental; these are a series of statistics that are in the testing phase and not yet fully developed.
We have published a blog post alongside this article that describes some of the challenges of estimating the prevalence of long COVID.
Nôl i'r tabl cynnwys2. Overview
This article presents a range of estimates relating to the number of people who experience symptoms beyond the acute phase of coronavirus (COVID-19) infection, commonly referred to as "long COVID". The estimates are expressed as percentages of the number of infected people in the study sample.
These estimates provide an indication of the likelihood of experiencing ongoing symptoms once infected with COVID-19. In contrast, the estimates reported in our monthly Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK bulletin are expressed out of everyone in the population, not just those infected. A further difference is that this analysis is based on laboratory-confirmed COVID-19 cases, whereas our monthly prevalence release includes both confirmed and suspected infections.
The initial sample for the analysis comprised 26,922 UK Coronavirus Infection Survey (CIS) participants who had a nose and throat swab that tested positive for COVID-19 using a polymerase chain reaction (PCR) test during study follow-up. This follow-up took place between 26 April 2020 (the beginning of the CIS) and 1 August 2021, irrespective of symptoms.
Because of the observational nature of this analysis, it is not possible to say with certainty whether symptoms reported after a positive test for coronavirus were caused by COVID-19 or something else.
Unless otherwise stated, our main interest throughout the article lies in symptom prevalence at four and 12 weeks (the thresholds used in the clinical case definitions of ongoing symptomatic COVID-19 and post-COVID-19 syndrome respectively) after the first infection.
The wide range of estimates provided in this experimental release emphasise that there is no universally agreed definition of long COVID, and different approaches to measuring its prevalence result in different estimates. We will continue to refine our estimates to take account of the scientific evidence as it evolves, collaborating with partners across academia, clinical practice, patient representatives and government, to develop and implement our research plans. For more information or to share your views on our plans, please email health.data@ons.gov.uk quoting "long COVID" in the subject line.
Nôl i'r tabl cynnwys3. Approach 1: Prevalence of any symptom at a point in time after infection
Symptoms analysed
Coronavirus (COVID-19) Infection Survey (CIS) participants are followed up weekly for the first month after enrolment and then approximately monthly for up to a year. At each visit, participants are asked whether they have experienced any of the following 12 symptoms in the past seven days: fever, headache, muscle ache, weakness/tiredness, nausea/vomiting, abdominal pain, diarrhoea, sore throat, cough, shortness of breath, loss of taste, and loss of smell. Parents and carers report symptoms on behalf of children aged under 12 years.
Follow-up intervals
We grouped CIS responses into 28-day intervals from the time of infection and calculated the percentage of participants who experienced any of the 12 symptoms out of all participants who responded during each interval. The time of infection was assumed to be midway between the date of the first positive polymerase chain reaction (PCR) test during CIS follow-up and the date of the previous negative test, up to a maximum lag of seven days back from the date of the first positive test.
It is possible for participants to respond more than once during a 28-day interval (for example, those on a weekly follow-up schedule or because of rescheduled monthly visits), so we used all visits within each interval when counting participants' symptoms.
Matching to a control group
All CIS participants are asked about the 12 symptoms at each follow-up visit, which enabled us to select a control group against which comparisons could be made. The control group comprised CIS participants who had never tested positive for infection or antibodies (where positive antibody test results obtained after COVID-19 vaccination were ignored). These tests took place either during study follow-up or outside of the study (for example, through national testing programmes or other surveillance studies), where the latter was self-reported on the questionnaire.
The analysis was based on 26,147 (97.1%) of the 26,922 CIS participants with a positive PCR test who could be matched to a control participant. Participants with COVID-19 were exactly one-to-one matched to control participants on factors that may be related to the likelihood of experiencing symptoms, measured at study enrolment: age (in years at last birthday), sex, and pre-existing health status (using the survey question "Do you have any physical or mental health conditions or illnesses lasting or expected to last 12 months or more, excluding any long-lasting COVID-19 symptoms?"). We included month and year of study enrolment and last visit in the matching set to ensure overlapping follow-up periods between participants with COVID-19 and matched controls.
For each control participant, symptom tracking began from their visit that was closest to that during which their matched participant tested positive for COVID-19 (to mitigate against the effect of any seasonal fluctuations in symptom reporting), less the same lag as that applied to the matched participant to identify the assumed date of infection. The median absolute difference in start-of-follow-up dates between participants with COVID-19 and their matched controls was six days, with a ninetieth percentile of 15 days.
Results
Among study participants with COVID-19, 9.4% reported any of 12 symptoms four to eight weeks after infection (based on responses from 15,061 participants), while 5.0% reported symptoms at 12 to 16 weeks (out of 12,611 participants) (Figure 1). These percentages were statistically significantly higher than in the control group, suggesting that the prevalence of symptoms following COVID-19 infection is greater than the background prevalence of these symptoms in the population at any given time. The difference in prevalence remained statistically significant at 20 to 24 weeks.
Figure 1: Approximately 1 in 20 study participants reported experiencing any of 12 common symptoms 12 to 16 weeks after COVID-19 infection
Percentage of study participants reporting any of 12 symptoms in four-week intervals from infection (participants with COVID-19) or from equivalent date (control participants), UK: 26 April 2020 to 1 August 2021
Embed code
Notes:
- "4 to 8 weeks" is the period spanning 29 to 56 days after infection, "8 to 12 weeks" is the period spanning 57 to 84 days after infection, and so on.
- The 12 symptoms comprise: fever, headache, muscle ache, weakness/tiredness, nausea/vomiting, abdominal pain, diarrhoea, sore throat, cough, shortness of breath, loss of taste, and loss of smell.
Download the data
The percentage of participants reporting symptoms four to eight weeks or 12 to 16 weeks after infection was greatest in females (10.7% and 5.4% respectively), adults aged 50 to 69 years (12.5% and 5.8% respectively), and people with a pre-existing health condition (13.9% and 7.4% respectively) (Table 1).
Each of these factors is considered in isolation without adjusting for the effect of others (for example, health status is related to age), so it is not possible to infer cause-and-effect relationships.
There was a clear gradient according to cycle threshold (Ct) (PDF, 806KB) value, which is the number of thermal cycles that a PCR test undergoes before a positive result is detectable. If there is a high quantity of viral genetic material present in the body (known as viral load), a positive result will be identified after few cycles. Participants testing positive with Ct values less than 23 had the highest symptom prevalence (12.0% at four to eight weeks and 5.5% at 12 to 16 weeks), while those with Ct values of 30 or higher had the lowest prevalence (6.8% at four to eight weeks and 4.4% at 12 to 16 weeks). Post-acute symptom prevalence was therefore highest in participants with signs of high viral load at the time of infection, but this may also be partly explained by the PCR test being taken soon after infection (and thus further from future follow-up visits), as viral load falls over time.
| Group | 4 to 8 weeks after infection | 12 to 16 weeks after infection | ||
|---|---|---|---|---|
| Participants with COVID-19 | Control participants | Participants with COVID-19 | Control participants | |
| All people | 9.4 (9.0-9.9) | 4.1 (3.8-4.4) | 5.0 (4.6-5.4) | 3.4 (3.1-3.8) |
| Males | 8.1 (7.5-8.8) | 3.7 (3.3-4.1) | 4.5 (4.0-5.1) | 3.3 (2.9-3.8) |
| Females | 10.7 (10.0-11.4) | 4.4 (4.0-4.9) | 5.4 (4.9-5.9) | 3.6 (3.1-4.0) |
| Age 2 to 11 years | 3.3 (2.5-4.5) | 3.6 (2.7-4.8) | 3.2 (2.3-4.5) | 4.1 (3.0-5.5) |
| Age 12 to 16 years | 4.6 (3.5-6.0) | 2.9 (2.1-4.0) | 3.0 (2.1-4.3) | 1.3 (0.8-2.3) |
| Age 17 to 24 years | 5.6 (4.4-7.1) | 3.6 (2.6-4.8) | 3.6 (2.5-5.1) | 3.6 (2.5-5.1) |
| Age 25 to 34 years | 7.6 (6.5-9.0) | 5.8 (4.8-7.1) | 4.5 (3.5-5.7) | 3.5 (2.7-4.7) |
| Age 35 to 49 years | 11.3 (10.2-12.4) | 5.0 (4.3-5.7) | 5.5 (4.7-6.4) | 4.5 (3.8-5.4) |
| Age 50 to 69 years | 12.5 (11.6-13.4) | 3.8 (3.3-4.4) | 5.8 (5.1-6.5) | 3.1 (2.7-3.7) |
| Age ≥70 years | 9.3 (8.1-10.8) | 2.8 (2.1-3.6) | 5.3 (4.3-6.5) | 3.1 (2.3-4.1) |
| Without health conditions | 8.6 (8.1-9.1) | 3.9 (3.6-4.3) | 4.5 (4.1-4.9) | 3.1 (2.8-3.5) |
| With health conditions | 13.9 (12.6-15.4) | 4.9 (4.1-5.8) | 7.4 (6.3-8.6) | 5.0 (4.1-6.0) |
| Cycle threshold <23 | 12.0 (11.1-13.0) | N/A | 5.5 (4.9-6.3) | N/A |
| Cycle threshold 23 to <30 | 10.7 (9.8-11.8) | N/A | 5.2 (4.5-6.0) | N/A |
| Cycle threshold ≥30 | 6.8 (6.2-7.4) | N/A | 4.4 (3.9-5.0) | N/A |
Download this table Table 1: Percentage of study participants (with 95% confidence intervals) reporting any of 12 symptoms four to eight weeks or 12 to 16 weeks after COVID-19 infection
.xls .csv4. Approach 2: Prevalence of continuous symptoms after infection
Definition of continuous symptoms
Analysing the duration of continuous symptoms from the assumed date of infection may provide stronger evidence for the presence of long COVID. In this analysis, "continuous symptoms" is taken to mean reporting any of the 12 symptoms at consecutive visits; it need not necessarily be the same symptom at every visit.
We tracked symptoms reported by participants on the day of their first positive test, or that started within the next five weeks. Unlike in other sections, for this analysis we used a post-acute threshold of five rather than four weeks so that the experience of participants on monthly follow-up schedules could be captured (it is common for the interval between monthly visits to be longer than 28 days).
The analysis was based on 20,565 (78.7%) of the 26,147 matched pairs of Coronavirus Infection Survey (CIS) participants described in Section 3 for which both participants in the pair had at least five weeks of CIS follow-up time after infection (or equivalent for matched controls). This was to ensure that participants had the opportunity to develop symptoms and thus be tracked in the analysis.
Symptom discontinuation was defined as the first occurrence of at least two successive follow-up visits without reporting any symptoms, to reflect the possibility of recurrent symptoms as widely reported in people experiencing long COVID.
Statistical techniques
Survival analysis techniques were used to estimate time from infection (or equivalent for matched controls) to symptom discontinuation (that is, when symptoms stopped during the study period).
Participants who did not experience symptom discontinuation during the study period were recorded as still experiencing symptoms on the date of the visit when symptoms were last reported; that is, their follow-up time was right-censored.
Follow-up time of study participants who experienced symptom discontinuation was interval censored. This means that the discontinuation was assumed to have occurred at an unknown time between the last visit at which symptoms were reported, and the first of two successive visits at which they were not.
Study participants who did not report symptoms on the day of their first positive test or within the next five weeks were recorded as having experienced symptom discontinuation on day zero.
Results
Among study participants who tested positive for COVID-19, 11.4% continued to report any of 12 symptoms for at least five weeks after infection, falling to 3.0% for at least 12 weeks (Figure 2). The corresponding estimates in the control group were statistically significantly lower, at 2.2% for at least five weeks and 0.5% for at least 12 weeks. These results suggest that the prevalence of symptoms that persist for at least five or 12 weeks is greater following COVID-19 infection than in the general population.
The 12-week estimates obtained from this analysis are lower than those reported in Section 3, particularly for the control group. This is because this analysis is based on experiencing symptoms over a continuous period rather than at a point in time (for the latter, there is no requirement for the participant to have previously experienced symptoms at any time).
Figure 2: Fewer than 1 in 30 study participants experienced any of 12 common symptoms continuously for at least 12 weeks after COVID-19 infection
Estimated percentage of study participants reporting any of 12 symptoms with time from infection (participants with COVID-19) or time from equivalent date (control participants), UK: 26 April 2020 to 1 August 2021
Embed code
Notes:
- For participants with COVID-19, day 0 is the assumed date of infection (midway between the date of the first positive test and the date of the previous negative test, up to a maximum lag of seven days back from the date of the first positive test).
- For control participants, day 0 is the date of their visit that was closest to that during which their matched participant tested positive for COVID-19, less the lag that was applied to their matched participant to find the assumed date of infection.
- The 12 symptoms comprise: fever, headache, muscle ache, weakness/tiredness, nausea/vomiting, abdominal pain, diarrhoea, sore throat, cough, shortness of breath, loss of taste, and loss of smell.
Download the data
Patterns in the prevalence of continuous symptoms across demographic groups (Table 2) were similar to those for point-in-time symptoms reported in Table 1. However, differences between participants with COVID-19 and matched controls were more pronounced.
Prevalence of persistent symptoms for at least five or 12 weeks after infection was greatest in females (13.1% and 3.4% respectively), adults aged 50 to 69 years (15.4% and 4.1% respectively), people with a pre-existing health condition (16.5% and 4.5% respectively), and those who tested positive with a cycle threshold (Ct) value less than 23 (15.8% and 3.9% respectively). As in the previous section, each of these factors is considered in isolation without adjusting for others, so it is not possible to infer cause-and-effect relationships.
Initial sample sizes and numbers remaining in the sample at five and 12 weeks post-infection (that is, those who were still experiencing symptoms and had not reached the end of the study period or been lost to follow-up) can be found in the data tables.
| Group | At least 5 weeks after infection | At least 12 weeks after infection | ||
|---|---|---|---|---|
| Participants with COVID-19 | Control participants | Participants with COVID-19 | Control participants | |
| All people | 11.4 (7.3-17.7) | 2.2 (0.9-5.3) | 3.0 (1.9-4.6) | 0.5 (0.2-1.1) |
| Males | 9.8 (5.8-16.7) | 2.0 (0.8-5.0) | 2.6 (1.5-4.6) | 0.4 (0.2-0.9) |
| Females | 13.1 (8.1-21.3) | 2.4 (1.0-5.8) | 3.4 (2.2-5.5) | 0.4 (0.2-0.7) |
| Age 2 to 11 years | 3.8 (1.6-9.2) | 2.1 (0.7-6.1) | 0.7 (0.3-2.0) | - |
| Age 12 to 16 years | 4.8 (2.3-9.8) | 1.1 (0.3-3.7) | 1.2 (0.5-2.7) | - |
| Age 17 to 24 years | 6.8 (3.1-15.0) | 2.1 (0.8-5.6) | 1.5 (0.7-3.3) | - |
| Age 25 to 34 years | 10.2 (5.9-17.6) | 2.8 (1.3-5.9) | 3.3 (1.4-7.5) | - |
| Age 35 to 49 years | 13.7 (8.0-23.5) | 2.8 (1.0-7.6) | 3.6 (2.1-6.2) | - |
| Age 50 to 69 years | 15.4 (9.3-25.6) | 2.1 (0.8-5.5) | 4.1 (2.2-7.6) | - |
| Age ≥70 years | 10.2 (5.3-19.5) | 1.5 (0.5-4.4) | 1.9 (1.0-3.5) | - |
| Without health conditions | 10.7 (6.5-17.8) | 2.2 (0.9-5.6) | 2.6 (1.6-4.1) | 0.3 (0.2-0.5) |
| With health conditions | 16.5 (9.8-27.9) | 3.0 (1.1-8.1) | 4.5 (2.5-8.0) | 0.6 (0.2-1.5) |
| Cycle threshold <23 | 15.8 (9.4-26.8) | N/A | 3.9 (2.3-6.7) | N/A |
| Cycle threshold 23 to <30 | 12.5 (6.8-23.0) | N/A | 2.7 (1.6-4.5) | N/A |
| Cycle threshold ≥30 | 7.6 (5.0-11.5) | N/A | 2.3 (1.2-4.1) | N/A |
Download this table Table 2: Estimated percentage of study participants (with 95% confidence intervals) reporting any of 12 symptoms continuously for at least five and 12 weeks after COVID-19 infection
.xls .csvPrevalence among symptomatic infections
All CIS participants are tested for COVID-19 at every follow-up visit, irrespective of symptoms. Our prevalence estimates are therefore applicable to everyone infected, not just those with symptoms at the acute phase of infection.
However, many other studies of long COVID prevalence are based on only symptomatic infections (see Section 6). To facilitate comparisons with these studies, we restricted our sample to the 43% of participants with COVID-19 who reported at least one of 12 common symptoms at the time of their first positive test or within the next five weeks.
Among study participants with symptoms at the acute phase, 27.0% continued to report symptoms for at least five weeks after infection (compared with 11.4% among all infections), and 6.7% for at least 12 weeks (compared with 3.0%). Confidence intervals accompanying these estimates can be found in the data tables.
Comparison with previously published estimates
In this analysis, we estimated that 11.4% and 3.0% of CIS participants with COVID-19 continued to report any of 12 common symptoms continuously for at least five and 12 weeks respectively, substantially lower the experimental statistics published in April 2021 based on a similar approach (21.0% at five weeks and 13.7% at 12 weeks). These changes in the estimates can largely be explained by having increased post-infection follow-up time among CIS participants, as well as methodological updates.
The CIS began as a pilot survey in April 2020 with the sample growing rapidly over the second half of the year, and just 117 participants in our sample tested positive for COVID-19 during the so-called "first wave" of infections in the UK. Our previous analysis used data to 6 March 2021, shortly after the peak of the "second wave" of infections. Taken together, these factors mean that participants' experience in terms of symptom duration was limited in our April analysis but is now clearer. Median post-infection CIS follow-up time increased from 80 days in our April 2021 analysis to 204 days in the present analysis. The proportion of participants with symptoms at the acute phase whose follow-up time was right-censored fell from 51% to just 3%.
Furthermore, in our previous analysis, 57% of participants with COVID-19 who had not experienced symptom discontinuation (two successive symptom-free visits) reported no symptoms at their last observed visit and were right-censored at this time. Some of these participants went on to be reclassified as having experienced symptom discontinuation according to our definition of two successive symptom-free visits.
The explanation above is illustrated in Figure 3. Participant A reported no symptoms at two successive follow-up visits (visits 2 and 3), so they experienced symptom discontinuation on the date of visit 2. Neither participants B nor C experienced two successive symptom-free visits, so their follow-up time was right-censored at the date of visit 3 (that is, they were treated as still having symptoms on this date) for our April 2021 analysis.
Participant D reported no symptoms at two successive follow-up visits (visits 3 and 4), so they experienced symptom discontinuation on visit 3. However, visit 4 took place after 6 March 2021 and had therefore not yet been observed in our April 2021 analysis. The follow-up time of this participant was right-censored at the date of visit 3 (last observed visit) in our April 2021 analysis, but the participant's outcome would later be reclassified as experiencing symptom discontinuation on the date of visit 3.
Figure 3: Follow-up visit sequences for four hypothetical study participants; dashed line represents the end-of-study date for our April 2021 analysis
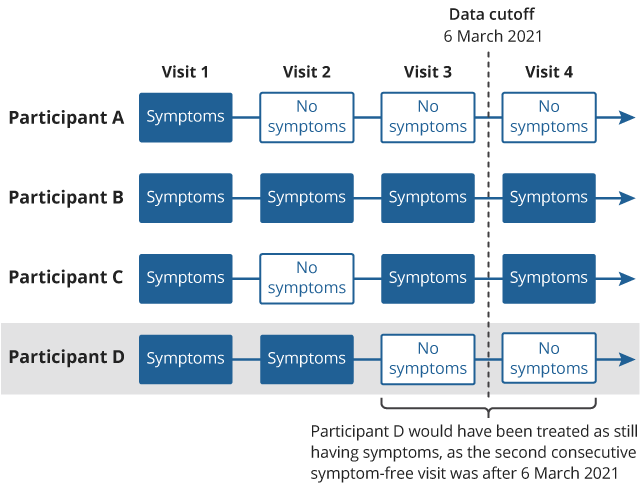
Download this image Figure 3: Follow-up visit sequences for four hypothetical study participants; dashed line represents the end-of-study date for our April 2021 analysis
.png (32.3 kB)For the present analysis, we have updated the methodology by right-censoring at the time of last observed symptoms rather than last visit, to mitigate against outcome misclassification bias which affected our previous estimates. A further methodological update comprises restricting the sample to participants who had at least five weeks of CIS follow-up time from the day of their first positive test (or equivalent date for matched controls), to ensure that participants had the opportunity to develop symptoms and thus be tracked in the analysis.
Analysis suggests that increased post-infection follow-up time among CIS participants was a large driver of the revision to previous estimates. Applying the same methods to the same study population as in our April 2021 release, but extending the follow-up time of these participants to 1 August 2021, resulted in prevalence estimates of 12.2% at five weeks and 3.3% at 12 weeks post-infection – similar to our revised estimates of 11.4% and 3.0% respectively.
Data and methodological changes mean that the estimates reported in this article are not comparable with those published in April 2021. Factors such as changes in clinical practice, COVID-19 variants and the national vaccination campaign may have also contributed to the change over time, but we are not able to confirm this from our analysis.
5. Approach 3: Prevalence of self-reported long COVID
Definition of self-reported long COVID
We evaluated the prevalence of self-reported long COVID four and 12 weeks after confirmed infection. In contrast with the previous sections, this analysis is based on self-classification of long COVID rather than tracking 12 specific symptoms.
Since 3 February 2021, the Office for National Statistics (ONS) has included the following question on its Coronavirus (COVID-19) Infection Survey (CIS): "Would you describe yourself as having 'long COVID', that is, you are still experiencing symptoms more than four weeks after you first had COVID-19, that are not explained by something else?"
Participants who answer positively to this question are then asked: "Does this reduce your ability to carry-out day-to-day activities compared with the time before you had COVID-19?" Possible responses comprise "Yes, a lot", "Yes, a little" and "Not at all".
These survey questions are also used to estimate the monthly population prevalence of self-reported long COVID. We used responses to these questions to estimate the trajectory of the likelihood of self-reported long COVID with time since infection. Unlike in Sections 3 and 4, this approach is based on self-classification of long COVID and thus reflects participants' perception of whether their lived experience is consistent with what they understand of the syndrome.
Statistical techniques
We performed a longitudinal analysis of follow-up visits from 3 February 2021 where time since infection was less than six months (as the sample was sparse for longer durations, which may have unduly influenced the estimates). The assumed date of infection was derived as reported in Sections 3 and 4.
The analysis was based on 21,374 (79.4%) of the 26,922 CIS participants with a positive polymerase chain reaction (PCR) test who responded to the survey questions on self-reported long COVID at least once since the questions were introduced. The nature of the survey questions meant that it was not possible to select a control group for this analysis.
The time trajectory of the likelihood of self-reported long COVID and activity-limiting self-reported long COVID was modelled using logistic regression, including all visits where the survey questions on long COVID were answered. The time trajectory was modelled as a restricted cubic spline with boundary knots at the fifth and ninety-fifth percentiles of time since infection and an interior knot at the median; see the data tables for results using alternative specifications.
Between 3 February 2021 and the end of the study period, participants responded to the survey questions on self-reported long COVID up to eight times, with a median of five responses per participant. We therefore estimated robust (clustered) standard errors to account for intra-participant correlation in survey responses.
Results
Among study participants who tested positive for COVID-19, 14.1% were estimated to be experiencing self-reported long COVID of any severity four weeks after infection, falling to 11.7% at 12 weeks (Figure 4). The estimated prevalence of self-reported long COVID resulting in at least some limitation to day-to-day activities was lower, at 9.3% at four weeks after infection and 7.5% at 12 weeks.
Figure 4: More than 1 in 10 study participants were experiencing self-reported long COVID 12 weeks after COVID-19 infection
Estimated percentage of study participants with self-reported long COVID with time from infection, UK: 26 April 2020 to 1 August 2021
Embed code
Download the data
The percentage of participants estimated to be experiencing self-reported long COVID four or 12 weeks after infection was greatest in females (16.0% and 13.4% respectively), adults aged 50 to 69 years (19.1% and 15.7% respectively), people with a pre-existing health condition (19.5% and 16.7% respectively), and those testing positive with Ct values less than 23 (19.2% and 15.5% respectively) (Table 3). As in previous sections, each of these factors is considered in isolation without adjusting for others, so it is not possible to infer cause-and-effect relationships.
| Group | Self-reported long COVID of any severity | Self-reported long COVID resulting in limitation to day-to-day activities | ||
|---|---|---|---|---|
| 4 weeks | 12 weeks | 4 weeks | 12 weeks | |
| All people | 14.1 (13.3-14.9) | 11.7 (11.3-12.1) | 9.3 (8.6-10.0) | 7.5 (7.2-7.9) |
| Males | 11.9 (10.9-13.1) | 9.8 (9.3-10.4) | 8.0 (7.1-9.0) | 6.2 (5.7-6.7) |
| Females | 16.0 (14.9-17.2) | 13.4 (12.8-14.0) | 10.4 (9.5-11.4) | 8.7 (8.2-9.2) |
| Age 2 to 11 years | 1.9 (1.1-3.4) | 1.7 (1.2-2.3) | 0.9 (0.4-2.0) | 1.0 (0.7-1.5) |
| Age 12 to 16 years | 4.7 (3.2-6.8) | 5.7 (4.7-6.9) | 2.4 (1.4-4.1) | 2.7 (2.0-3.5) |
| Age 17 to 24 years | 8.6 (6.5-11.4) | 8.5 (7.2-9.9) | 5.6 (3.8-8.1) | 4.2 (3.3-5.4) |
| Age 25 to 34 years | 13.5 (11.4-16.0) | 10.3 (9.2-11.5) | 6.6 (5.1-8.5) | 5.7 (4.9-6.7) |
| Age 35 to 49 years | 18.6 (16.8-20.5) | 14.3 (13.3-15.2) | 12.3 (10.8-14.0) | 8.9 (8.1-9.6) |
| Age 50 to 69 years | 19.1 (17.5-20.8) | 15.7 (14.9-16.5) | 13.3 (12.0-14.8) | 10.8 (10.1-11.5) |
| Age ≥70 years | 10.2 (8.3-12.4) | 10.1 (9.0-11.3) | 7.7 (6.1-9.7) | 7.6 (6.6-8.6) |
| Without health conditions | 13.0 (12.2-13.9) | 10.6 (10.2-11.1) | 8.1 (7.4-8.8) | 6.5 (6.1-6.8) |
| With health conditions | 19.5 (17.4-21.7) | 16.7 (15.5-17.9) | 14.9 (13.1-17.0) | 12.5 (11.5-13.5) |
| Cycle threshold <23 | 19.2 (17.6-21.0) | 15.5 (14.6-16.3) | 12.7 (11.3-14.3) | 9.8 (9.2-10.5) |
| Cycle threshold 23 to <30 | 15.6 (13.8-17.4) | 12.1 (11.2-13.0) | 11.1 (9.6-12.8) | 7.8 (7.1-8.5) |
| Cycle threshold ≥30 | 10.8 (9.9-11.9) | 9.0 (8.5-9.6) | 6.7 (5.9-7.6) | 5.9 (5.4-6.4) |
Download this table Table 3: Estimated percentage of study participants (with 95% confidence intervals) with self-reported long COVID of any severity or with activity limitation, at four and 12 weeks after COVID-19 infection
.xls .csvThe estimated percentage of participants with self-reported long COVID 12 weeks after infection (11.7%) is notably higher than that of participants with symptoms persisting for at least 12 weeks reported in Section 4 (3.0%).
The self-reported long COVID response may better capture the relapsing nature of long COVID symptoms. Of participants who were classed as experiencing symptom discontinuation (two successive symptom-free visits) in our survival analysis approach in Section 4, 14.1% reported at least one of the 12 symptoms in a subsequent follow-up visit. Some of these participants may still describe themselves as having long COVID in our analysis in Section 5 if, from their past experience, they expect periods of improvement to be followed by relapse.
Furthermore, the percentage of participants with symptoms persisting for at least 12 weeks using the survival analysis approach increased to 6.1% when we defined symptom discontinuation as being three rather than two successive symptom-free visits (see the data tables) for full sensitivity analysis results).
It is also likely that the 12 symptoms included in the survival analysis approach do not include some symptoms often associated with long COVID, which may further explain the disparity between the estimates.
Prevalence among symptomatic infections
Among study participants with symptoms at the acute phase, 22.3% were estimated to be experiencing self-reported long COVID of any severity four weeks after infection (compared with 14.1% among all infections), and 17.7% at 12 weeks (compared with 11.7%).
When focussing on activity-limiting long COVID, 15.1% were estimated to be experiencing self-reported long COVID four weeks after infection (compared with 9.3% among all infections), and 11.8% at 12 weeks (compared with 7.5%).
Confidence intervals accompanying these estimates can be found in the data tables.
Nôl i'r tabl cynnwys6. Comparison with other studies
Various studies into the prevalence of post-acute symptoms have resulted in a wide range of estimates, each study having its own long COVID definition, study population and design, data collection instrument and statistical methodology. As these studies are generally based on symptomatic coronavirus (COVID-19) infections, in this section we compare estimates from several large UK studies with those from our own analysis, relating to participants who had symptoms at the acute phase of infection (within the first five weeks).
COVID Symptom Study
Our estimates of the percentage of study participants with symptomatic COVID-19 who experience at least one of 12 symptoms continuously for at least five weeks (27.0%) or 12 weeks (6.7%) are greater than those reported by the COVID Symptom Study (13.3% at four weeks and 2.3% at 12 weeks). Like our survival analysis results reported in Section 4, the COVID Symptom Study estimates are based on a prospective symptom tracking approach, using daily data on symptoms reported by mobile phone application users who had previously tested positive by polymerase chain reaction (PCR) test.
UK National Core Studies – Longitudinal Health and Wellbeing programme
We estimated that 22.3% of study participants with symptomatic COVID-19 would describe themselves as having long COVID four weeks after infection, and 17.7% 12 weeks after infection. Slightly lower results were estimated by the UK National Core Studies -- Longitudinal Health and Wellbeing programme, which analysed data from 10 longitudinal studies of various designs, sample sizes and age ranges. These studies use retrospective reporting of symptom duration following confirmed or suspected infection. Estimates across the 10 studies ranged from 14.5% to 18.1% at four weeks after infection, and 7.8% to 17.0% at 12 weeks. When analysing studies that focussed solely on severe long COVID, the range of estimates decreased to 3.0% to 13.7% at four weeks after infection, and 1.2% to 5.4% at 12 weeks, again lower than our estimates relating to activity-limiting, self-reported long COVID of 15.1% at four weeks and 11.8% at 12 weeks.
REACT-2
All of the percentages reported in this article are lower than those from the REACT-2 study, which found that 37.7% of people continue to experience at least one symptom 12 weeks after confirmed or suspected COVID-19 infection, based on retrospective reporting of the duration of 29 individual symptoms. This estimate may be higher than our own because it includes suspected infections and is based on an extensive list of symptoms, some of which may be common in the general population at any given time (such as a runny nose and sneezing).
PHOSP-COVID
Our estimates are lower than those from the PHOSP-COVID study, in which 71% of participants did not feel fully recovered at a median of five months after hospital discharge. However, all PHOSP-COVID study participants were hospitalised with COVID-19 and were therefore likely to have generally experienced more severe acute illness compared with participants in our study, 2% of whom reported to have been hospitalised with COVID-19.
Nôl i'r tabl cynnwys8. Glossary
Coronavirus and COVID-19
Coronaviruses are a family of viruses that cause disease in people and animals. They can cause the common cold or more severe diseases, such as COVID-19. COVID-19 is the name used to refer to the disease caused by the SARS-CoV-2 virus, which is a type of coronavirus. The Office for National Statistics (ONS) takes COVID-19 to mean presence of SARS-CoV-2 with or without symptoms.
First and second waves
There are no universally agreed definitions of the "first wave" and "second wave" of infections. In this analysis, we define the first wave as starting on 24 January 2020, when the first confirmed coronavirus (COVID-19) case was reported in the UK, and the second wave as starting on 21 August 2020, when the COVID-19 reproduction number (R) in England increased to above 1 for the first time since it was first reported on 22 May 2020.
Survival analysis
Survival analysis comprises a collection of statistical techniques that are appropriate for analyses where the quantity of interest is the time until an event occurs (despite the name, this event does not have to be death). In this analysis, the event of interest was discontinuation of symptoms. A key feature of survival analysis is that for some participants, the event of interest does not occur within the study period; the follow-up time of these participants is said to be "censored", and this censoring is taken into account when estimating the average time until the event occurs.
Nôl i'r tabl cynnwys9. Data sources and quality
This analysis was based on data from the Coronavirus (COVID-19) Infection Survey (CIS). Further information can be found in the CIS methodology guide, study protocol and survey questionnaire.
Strengths
The CIS comprises a large sample of private households randomly sampled from the UK population (excluding communal establishments such hospitals, care homes, schools, halls of residence and prisons). All household members aged two years or over from sampled households are invited to enrol. Since the CIS began in April 2020, over 476,000 individuals from over 236,000 households have participated in the study.
All CIS participants, including those who do not have COVID-19 or who carry the virus but have no symptoms, are asked to provide swab samples at every follow-up visit. This analysis is therefore applicable to all people with COVID-19, not just those with symptoms during the acute phase of infection.
Limitations
Like all household surveys, not all sampled households who are invited to participate in the CIS enrol. This analysis is based on unweighted data without imputation for non-response, so bias will be introduced if response rates are related to personal characteristics that are also related to symptom duration following COVID-19 infection.
The initial CIS sample for England, selected in April 2020, achieved a response rate of 51%. More recently, the response rate has dropped to 13%; this may be because the initial sample came from households who had already participated in other Office for National Statistics (ONS) surveys and had agreed to be approached for future research. After this, addresses were selected at random from address lists. Detailed information on response rates, including for other countries of the UK, which joined the study later than England, can be found in Tables 2a to 2f of the technical datasets accompanying the latest CIS statistical bulletin.
Participants might also miss scheduled visits or drop out of the study after enrolment. Bias may be introduced if missed visits and loss-to-follow-up are related to long COVID, for example participants being more willing, or less able, to continue in the study because of their symptoms. Matching on month and year of last visit ensured similar loss-to-follow-up rates (no contact in the 12 weeks before the end of the study period) between participants with COVID-19 and matched controls (both 12%). Loss-to-follow-up rates were highest among participants aged 17 to 34 years (Table 4).
| Group | Participants with COVID-19 | Control participants |
|---|---|---|
| All people | 12.4 | 11.9 |
| Males | 12.6 | 12.0 |
| Females | 12.1 | 11.8 |
| Age 2 to 11 years | 11.3 | 10.4 |
| Age 12 to 16 years | 9.4 | 8.8 |
| Age 17 to 24 years | 19.9 | 19.6 |
| Age 25 to 34 years | 17.8 | 17.3 |
| Age 35 to 49 years | 11.5 | 11.2 |
| Age 50 to 69 years | 10.3 | 9.7 |
| Age ≥70 years | 10.3 | 9.6 |
| Without health conditions | 13.0 | 12.5 |
| With health conditions | 8.8 | 8.2 |
| Cycle threshold <23 | 12.2 | N/A |
| Cycle threshold 23 to <30 | 11.9 | N/A |
| Cycle threshold ≥30 | 12.9 | N/A |
Download this table Table 4: Estimated percentage loss-to-follow-up among participants with COVID-19 and matched control
.xls .csvSelf-reported measures are subjective and reflect systematic differences between socio-demographic groups. This can be in terms of their likelihood to report symptoms given an underlying level of severity, as well as differences in severity. If participants are more likely to report symptoms following a positive test for COVID-19, because of increased awareness of long COVID, the estimated difference in symptom duration between participants with COVID-19 and matched controls will be exaggerated.
The 12 symptoms in our survival analysis approach do not include all those often associated with long COVID such as heart palpitations, chest pain, sleep disruption, cognitive impairment, and depression and anxiety. This may have downwardly biased the estimates. We are exploring options for adding additional symptoms to the CIS questionnaire to address this gap.
The CIS began as a pilot survey in April 2020 and the sample size grew rapidly over the second half of the year. In the absence of widespread community testing during the first wave of the pandemic, many people infected during this period may not have received a positive test. Some of these people may be included in our control group, and thus differences in prevalence estimates between participants with COVID-19 and matched controls can be considered conservative in this respect.
Nôl i'r tabl cynnwys